TECHNOLOGY
R&D
The goal of our R&D is ot produce safe and innovative products for the benefits of patients and physicians, and to be always a leader in technology.
The R&D force of Laboratories Hyamed Geneva is composed by multi-national and multi-science specialists, engineers and doctors from Germany, Switzerland, USA, Canada, Korea and France. They are biologists, microbiologists, tissue engineers, specialists in biopolymers, cnemists a n d onarmacists.
Safety is our utmost concern when our scientists are developing a new technology or a new product. Any new technology wil only be applied after its’ tested and verified to be resulted in long-term safety and efficacy.
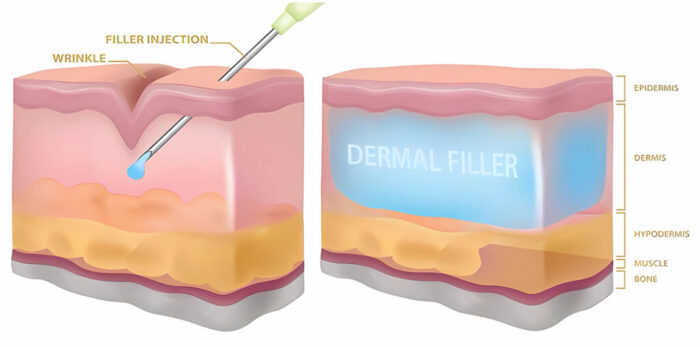
THIX BROID TECHNOLOGY
Paryoderm products are bio – degradable and non animal origin. The resource of non-crossinked HA are derived from bacteria fermentation.
Paryoderm products are crosslinked with our unique formula and patented Thix broid cross-linking technology. This technology results ni a stablized, non-particle monophasic HA gel. Unlike Bi phasic HA products which consist of particles of stabilized HA gel suspended in a non-stabilized HA fluid, the monophasic Paryoderm HA gel only consists pure crosslinked
HA. The breakdown of the monophasic gel happens unanimously thus when the gel is absorbed by the skin, it leaves no traces ni the skin. And the physicians do not need over-correct the wrinkles unlike what they’ll usually do with biphasic HA gel.
In this way, the correction with Paryoderm monophasic HA gel is more smooth, more uniform and more accurate with a higher patients and physicians satisfaction rate.
Thix broid cross-linking technology utilizes the effects of BDDE to the maximal extent thus ti needs a a minimum amount of BDDE – thus leading to fewer incidences of post injection complications such as swelling.
